The Triiodomethane (Iodoform) Reaction
- Page ID
- 3921
This page looks at how the triiodomethane (iodoform) reaction can be used to identify the presence of a CH3CO group in aldehydes and ketones. There are two apparently quite different mixtures of reagents that can be used to do this reaction. They are, in fact, chemically equivalent.
Using iodine and sodium hydroxide solution
This is chemically the more obvious method. Iodine solution is added to a small amount of aldehyde or ketone, followed by just enough sodium hydroxide solution to remove the color of the iodine. If nothing happens in the cold, it may be necessary to warm the mixture very gently. A positive result is the appearance of a very pale yellow precipitate of triiodomethane (previously known as iodoform) - CHI3. Apart from its color, this can be recognised by its faintly "medical" smell. It is used as an antiseptic on the sort of sticky plasters you put on minor cuts, for example.
Using potassium iodide and sodium chlorate(I) solutions
Sodium chlorate(I) is also known as sodium hypochlorite. Potassium iodide solution is added to a small amount of aldehyde or ketone, followed by sodium chlorate(I) solution. Again, if no precipitate is formed in the cold, it may be necessary to warm the mixture very gently. The positive result is the same pale yellow precipitate as before.
The chemistry of the triiodomethane (iodoform) reaction
A positive result - the pale yellow precipitate of triiodomethane (iodoform) - is given by an aldehyde or ketone containing the grouping:
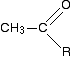
"R" can be a hydrogen atom or a hydrocarbon group (for example, an alkyl group). If "R" is hydrogen, then you have the aldehyde ethanal, CH3CHO.
- Ethanal is the only aldehyde to give the triiodomethane (iodoform) reaction.
- If "R"is a hydrocarbon group, then you have a ketone. Lots of ketones give this reaction, but those that do all have a methyl group on one side of the carbon-oxygen double bond. These are known as methyl ketones.
Equations for the triiodomethane (iodoform) reaction
We will take the reagents as being iodine and sodium hydroxide solution. The first stage involves substitution of all three hydrogens in the methyl group by iodine atoms. The presence of hydroxide ions is important for the reaction to happen - they take part in the mechanism for the reaction.

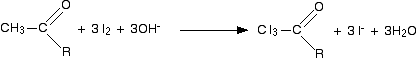
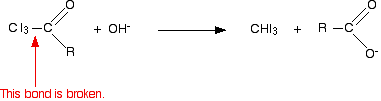
In the second stage, the bond between the C I3 and the rest of the molecule is broken to produce triiodomethane (iodoform) and the salt of an acid.

Putting all this together gives the overall equation for the reaction:

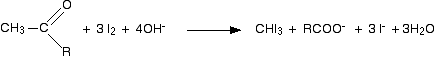
Contributors
Jim Clark (Chemguide.co.uk)


