Natural Occurrence of Aldehydes and Ketones
- Page ID
- 744
Aldehydes and ketones are widespread in nature and are often combined with other functional groups. Examples of naturally occurring molecules which contain a aldehyde or ketone functional group are shown in the following two figures. The compounds in the figure 1 are found chiefly in plants or microorganisms and those in the figure 2 have animal origins. Many of these molecular structures are chiral.
When chiral compounds are found in nature they are usually enantiomerically pure, although different sources may yield different enantiomers. For example, carvone is found as its levorotatory (R)-enantiomer in spearmint oil, whereas, caraway seeds contain the dextrorotatory (S)-enantiomer. In this case the change of the stereochemistry causes a drastic change in the perceived scent. Aldehydes and ketones are known for their sweet and sometimes pungent odors. The odor from vanilla extract comes from the molecule vanillin. Likewise, benzaldehyde provides a strong scent of almonds and is this author’s favorite chemical smell. Because of their pleasant fragrances aldehyde and ketone containing molecules are often found in perfumes. However, not all of the fragrances are pleasing. In particular, 2-Heptanone provides part of the sharp scent from blue cheese and (R)-Muscone is part of the musky smell from the Himalayan musk deer. Lastly, ketones show up in many important hormones such as progesterone (a female sex hormone) and testosterone (a male sex hormone). Notice how subtle differences in structure can cause drastic changes in biological activity. The ketone functionality also shows up in the anti-inflammatory steroid, Cortisone.
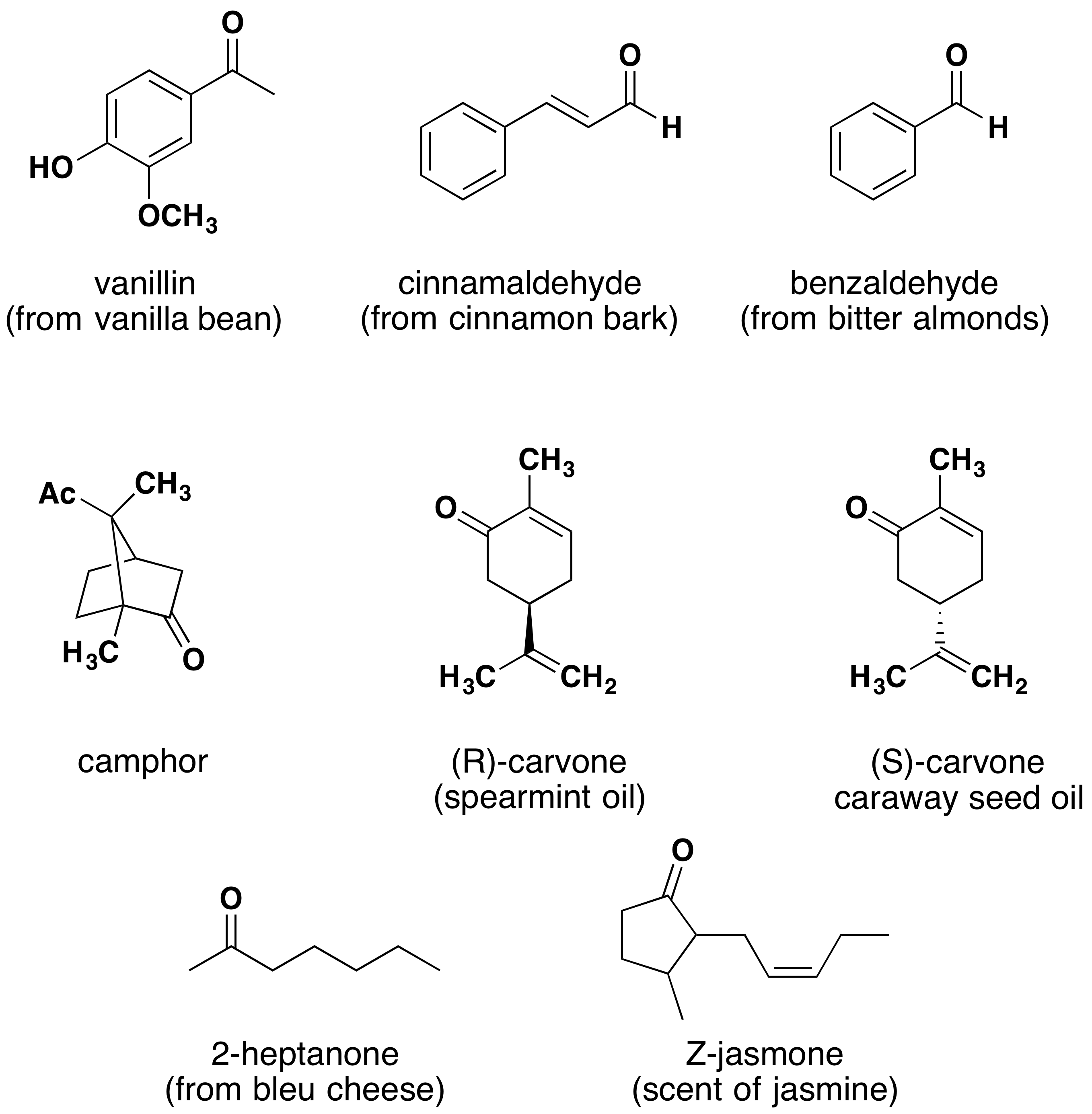
Figure 1. Aldehyde and ketone containing molecules isolated from plant sources.
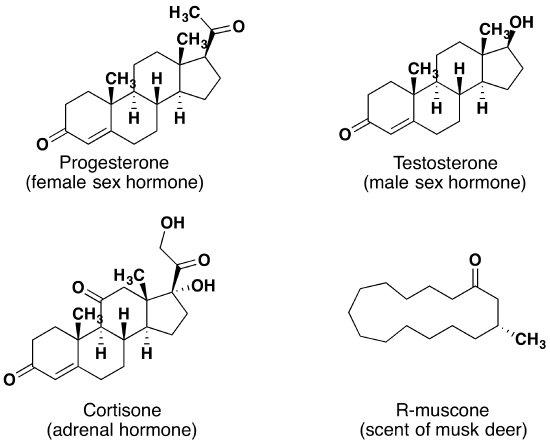
Figure 2. Aldehyde and ketone containing molecules isolated from animal sources.
Contributors
Prof. Steven Farmer (Sonoma State University)
William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry

