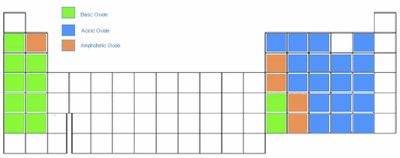
Acid-Base Character of Oxides and Hydroxides
- Page ID
- 68247
From left to right on the periodic table, acid-base character of oxides and hydroxides go from basic to acidic.
- Increasing charge on an anion increases the production of basic solutions.
- As electronegativity increase, production of ionic cations increases because elements are more able to adopt a cation.
- As ionization energy increases, the acidic nature increases.
Metallic Oxides:
- Ionic Bonding: no distribution of electron wave function
- Ionic oxides are usually basic (element act as a base when reacting with H2O)
Na2O(s) + H2O(l) --> 2NaOH(aq) --> 2Na+(aq) + 2OH-(aq)
B. Oxide B. Hydroxide
Semimetal Oxides:
- Semimetal are amphoteric (elements acts as an acid and/or base when reacting depending on pH of solution)
Al2O3 --> Al(OH)3 --(3H+)--> [Al(H2O)6]^(3+) (aq)
--(OH-)--> [Al(OH)4]-(aq)
Non-Metal Oxides
- Covalent Bonding: almost complete distribution of electron wave function
- Covalent oxides are usually acidic (elements act as an acid when reacts with H2O)
SO3 + H2O(l) -> H2SO4(aq) -> H+ + HSO4-
A. Oxide A Hydroxide
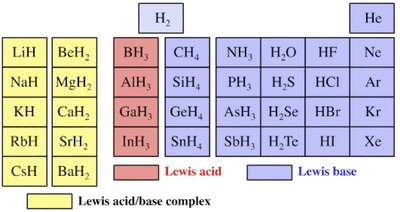
Ionic Hydrides
- Ionic Bonding: no distribution of electron wave function
- Bronsted Basic because they will react with proton
- Lewis Basic because they can be ligands
CaH2 + 2H2O -> 2H2 + Ca(OH)2
H- H+ H2
-In this case, CaH2 is basic because it reacts with water (an acid in this case) to form many hydrides by reducing a proton.
Covalent Hydrides
- Covalent Bonding: almost complete distribution of electron wave function
HF + H2O -> F- + H3O+ ....can also be written as HF(aq) <--> H+(aq) + F-(aq)
H+ H+ H+
- HF is a weak acid that is bronsted acid because it will loose a proton. Therefore, HF is the weak acid, where the water acts as a silent water, and F- is the weak conjugate base.